Get 1000 Hours free
On the UCSD Supercomputer
Start Your Trial
Bioinformatics and functional selection of GH77 4-α-glucanotransferases for potato starch modification
Christensen et al. (2024) New Biotechnology 79, 39-49.
Along with cellulose and lignin, starch is among the most abundant biopolymers on the planet. It is the most important source of nutrition for much of earth’s human population. In addition to its role in simply providing caloric sustenance to human populations, starch also has commercial importance in food preparation and presentation. Starch polymers are found in many foods, from gummies to salad dressing to infant formula, and are important in giving products specific textures and improved flavor. Starches are important in a variety of non-food uses as well; they are used as drilling lubricants and in paper and textile preparation.
Much effort has been dedicated to developing techniques to modify starch polymers to further improve their characteristics and expand their application in food technology. The increased consumer interest in eating less processed foods (i.e. the so-called white-label foods), has created a growing market for techniques that modify starches through enzymatic treatment. Because enzymatic modifications avoid “chemical” processing (i.e. treatment with non-biological compounds) they have added value in the current marketplace for food additives.
There is now a growing set of techniques in the food industry centered on the use of glycolytic enzymes that can improve starch properties for food applications at industrial scales. One important goal is to make starch gels more similar to gelatin in consistency and feel. (Since gelatin is an animal product, it is unacceptable for certain consumers). To understand the overall strategy, it is important to know that “starch” consists of two distinct polymers, amylose (which makes up 20-30%) and amylopectin (which makes up 70-80%). Amylose is a linear molecule consisting almost exclusively of α-1,4-linked glucose residues, while amylopectin is branched, and contains α-1,6-linked branch points added to linear chains of α-1,4-linked glucose. When starch is heated in water, gelatinization occurs, creating a viscous paste. When cooled, the gelatinized starch forms a textural gel network if the components are at a critical concentration. As part of this process, amylose forms connected double-helix aggregates quickly, followed by amylopectin gelation, which happens more slowly.
One of the key enzymatic activities that alter gelation properties is microbial 4-α-glucanotransferase activity (4αGTs, EC 2.4.1.25) from the glycoside hydrolase family 77 (GH77). 4-α-glucanotransferase enzymes influence gelation by adding linear amylose chains to the branched amylopectin. The reaction decreases the amount of amylose present in starch and increases amylopectin branching. Under the correct conditions, the process can improve the viscosity of the starch gel. As a result, there is considerable interest in identifying 4-α-glucanotransferase enzymes that have potential for industrial food applications. For commercial use, activities from thermophiles are particularly valued, and the industry standard currently is the 4-α-glucanotransferase from Thermus thermophilus.
The question is, are there other 4-α-glucanotransferase enzymes that might perform better, or differently, making it possible to develop starch gels that have novel physical properties. To explore this possibility, Christensen et al. performed an extensive study of microbial 4-α-glucanotransferase enzymes in a search for potential new activities. To initiate the study, the authors examined all 12000+ 4-α-glucanotransferase sequences from the Conserved Unique Peptide Patterns (CUPP) database, first de-duplicating it, and eliminating sequences that were too short or too long to be relevant. The resulting set of 6,229 sequences were aligned using Muscle, then the authors created an all-inclusive 4-α-glucanotransferase tree using IQ-Tree on CIPRES. The resulting tree is shown in Figure 1 below (reproduced under Creative Commons license). The known 4-α-glucanotransferase enzymes fall into 4 specific classes, A, B, C, and D. Class A enzymes have a single catalytic domain, while Class B and C have a single catalytic domain with an additional N-terminal domain. In Classes C and D, the catalytic domain is interrupted by a domain of unknown function. The authors selected 27 sequences from classes A, B, and C, cloned and expressed them in E. coli, and studied the enzymatic activities of the expressed enzymes.
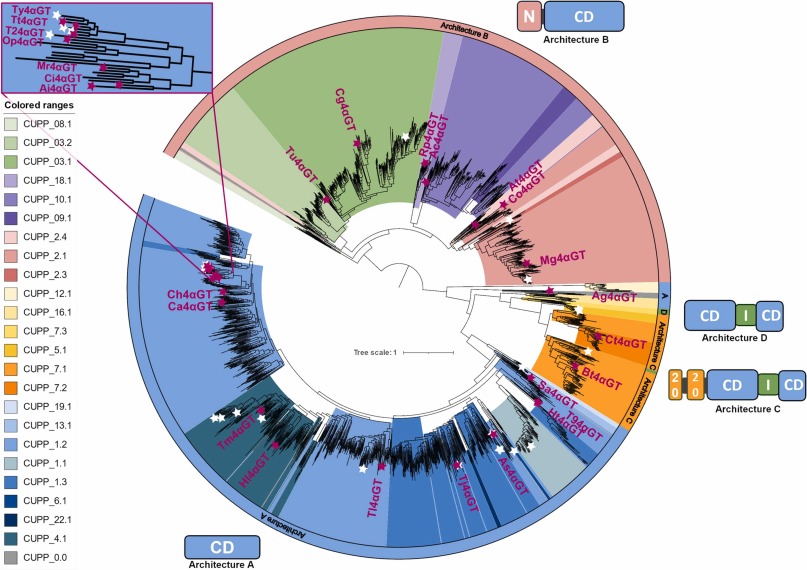
Figure 1. The maximum likelihood tree based on 2190 GH77 protein sequences. Color ranges indicate CUPP groups. The legend is ordered according to the tree-order. The different colors around the circle's edge represent different enzyme domain architectures. Catalytic domains (CD), interrupting domains of unknown function (I), N-terminal domains (N), and CBM20s (20) are indicated in domain architecture figures. White stars represent previously characterized GH77 4αGTs (Suppl. Table S2). Pink stars represent GH77 members selected for screening in this study, with enzyme names indicated in pink text. Reproduced from Christensen et al (2024), under Creative Commons license.
The 27 sequences selected for further study represent 12 distinct CUPP groups, and one enzyme was not a member of any known CUPP group. Particular focus was given to the enzymes closely related to the commercial enzyme from Thermus. Accordingly, six enzymes from this group were cloned for study. The activity of all 27 enzymes toward potato starch was quantified, and the authors selected five of the novel enzymes with very high starch modifying activity for more detailed study. Two of the highly active enzymes had very low sequence similarity to the commercial Thermus enzyme, while the remaining three were closely related to Thermus. The detailed studies examined starch modifying activity as a function of temperature and pH, and the impact of activity on starch gelation properties over time was evaluated.
The authors found that while 4-α-glucanotransferases are broadly distributed across microorganisms, those with desirable characteristics for commercial applications (i.e. high activity towards potato starch and thermostability), are restricted to enzymes in Class A,with a single catalytic domain. The authors note that the simpler architecture of A enzymes may be required for thermostability. One of the enzymes investigated in further detail had potentially more desirable properties than the Thermus thermophilus enzyme, while two of the most active enzymes were in entirely different, and more distantly related CUPP groups. The latter two represent opportunities to search for new and potentially valuable sources of commercial enzymes.
The study also reports a model for starch gelation where recrystallization is triggered by formation of amylose-amylose double-helixes initially and the gel is strengthened by later formation of amylopectin-amylopectin and amylose-amylopectin helixes. The gelation properties depends upon the relative content of amylose and amylopectin, as well as the length of the respective polymers. While native potato starch does not form strong gels, brief treatments with 4-α-glucanotransferases allows starch to form a strong gel, as transferring amylose to amylopectin creates a stronger gel matrix.
